
Three reports (see references below) provide evidence that the mitochondrial channel, VDAC, can act as an insertion catalyst. More precisely, VDAC is responsible for a process termed, " Auto-Directed Insertion". Using mutants with asymmetric voltage-gating properties, Zizi et al. (1995) showed that, in multi-channel membranes, virtually all the channels inserted in the same direction as monitored by measuring the asymmetry of the voltage dependence. However, the direction of insertion was apparently random from membrane to membrane and unaffected by the membrane potential. The conclusion was that the first channel inserted in a random direction and virtually all subsequent channels followed suit. Considering the target size of one channel as opposed to that of the entire planar phospholipid membrane, a catalytic acceleration of 109 was estimated.
Direct evidence that VDAC channels in the membrane are the sites of insertion, was obtained by examining, in detail, the ability of urea and guanidinium chloride (GdmCl) to accelerate the rate of VDAC insertion into planar membranes (Xu & Colombini, 1996). These agents can increase the insertion rate by as much as 60 fold when added to the aqueous phase on the side opposite to that to which the detergent-solubilized VDAC channels were added. This effect is rapidly reversible by washing out the urea or GdmCl and inhibited by sarcosine, a substance that makes proteins more rigid. Osmotic and salt effects were eliminated as underlying causal factors (Xu & Colombini, 1996). Therefore urea and GdmCl were proposed to induce discrete structural changes in VDAC that caused VDAC to be a better insertion catalyst. Most importantly, these experiments provided strong evidence that VDAC channels insert at the site of the VDAC channels already in the membrane as the trivial amounts that could conceivably cross the phospholipid membranes could not possibly affect the channels trying to insert into the membrane.
A flavor of the experimental results follows:
The experimental set-up is a planar phospholipid membrane generated in a 0.1 mm hole in a plastic partition. This membrane separates 2 aqueous compartments, labeled cis and trans. See the chamber schematic:
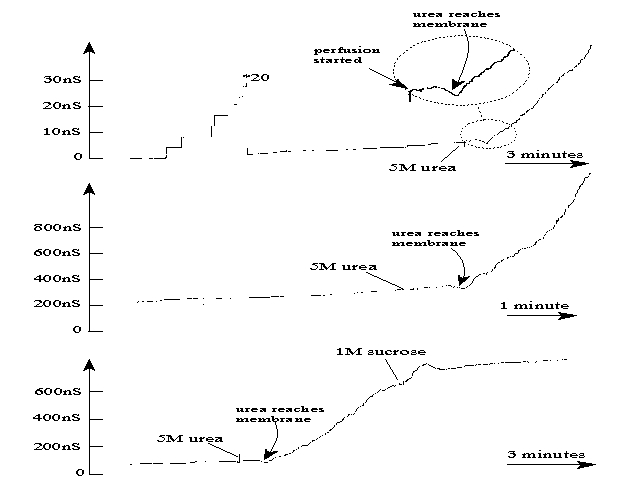
An example of the accelerated insertion is shown in the next figure:
A sample of VDAC from N. crassa was added to the trans compartment
and channels insert after a time (stepwise increases in current in upper
panel). When the cis compartment was perfused with 5M urea (also contained
1 M KCl and buffer) by displacing the lighter solution present (1M KCl
plus buffer) one can see when the urea solution reaches the level of the
membrane because it causes a drop in the conductance of the channels already
in the membrane. Almost at the same time, the rate of channel insertion
increases dramatically (upper and middle panels). In the lower panel,
after the urea effect was established, the urea was removed by displacing
it with a sucrose solution (also contained KCl and buffer). Note that
the rate of channel insertion returned to the level seen prior to perfusion
with urea.
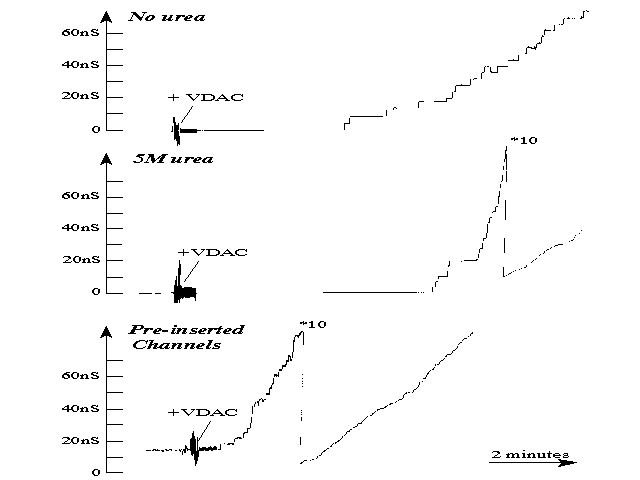
Further evidence was provided by showing that VDAC, pre-inserted into
the membrane, reduced the insertion lag time (Xu & Colombini, 1996).
In the figure, the upper panel and the middle panels show long delays
prior to insertion of VDAC. The presence of 5M urea on the opposite side
(middle panel) did increase the rate of insertion but not the delay to
insertion. Thus, the first insertion event was not catalyzed. However,
the lower panel shows that in the presence of a few channels already in
the membrane there is only minimal insertion delay consistent with that
expected from an unstirred layer.
References
Zizi, M., Thomas, L., Blachly-Dyson, E., Forte, M. and Colombini, M. 1995. Oriented channel insertion reveals the motion of a transmembrane beta strand during voltage gating of VDAC. Journal of Membrane Biology, 144:121-129.
Xu, X., and Colombini, M. 1996. Self-catalyzed insertion of proteins into phospholipid membranes. Journal of Biological Chemistry, 271: 23675-23682.
Xu, X. and Colombini, M. 1997. Auto-directed insertion: pre-inserted VDAC channels greatly shorten the delay to the insertion of new channels. Biophysical Journal, 72: 2129-2136.
Please
send comments and contributions to Dr.
Marco Colombini |
Last modified: March 27, 2004 15:28 Page created and maintained by Swapnil Sharma |