The concept of hybridization provides a way to think about the orbitals and bonding in organic molecules. Without hybridization, for example, it would be difficult to rationalize how methane, a molecule that is perfectly tetrahedral with identical C-H bonds, could be formed from an s and 3 p orbitals. Rather, we think about methane as utilizing four equivalent sp3 hydrid orbitals on C. The "raw materials" for hybrid orbitals are the s and p atomic orbitals (AO's); the s and p AO's are mixed (mathematically) to form hybrid orbitals.
The number of new (hybrid) orbitals is equal to the number of AO's mixed together to form the hydrid orbitals. For example, mixing an s and three p's give four new sp3 hybrid orbitals. One need not use all of the AO's (the p's) in the hybridization process (the hybridization of B in BH 3 is sp2 ; the remaining p orbital is unused). Any unutilized p's can be used to form p- bonds (or they not be used at all for bond formation). The hybrid orbitals form s-bonds.
| hydridize | hybrid orbitals | # p's left over | # s bonds | # p bonds | shape | |
| #s | #p | |||||
| 1 | 1 | 2 sp | 2 | 2 | 2 | linear |
| 1 | 2 | 3 sp2 | 1 | 3 | 1 | trig planar |
| 1 | 3 | 4 sp3 | 0 | 4 | 0 | tetrahedral |
The "first" bond between two atoms is a s-bond formed from the head-on overlap of orbitals. The bonding e-density (orbital density) in a s- bond is concentrated in the region between the two nuclei that are joined by the bond. If there are second and third bonds between the atoms, these are p- bonds and formed from the side-to-side overlap of p-orbitals. The electron density in a p- bond is on opposite sides of the line between the two nuclei joined by the bond. The number of p- bonds an atom forms automatically determines its hybridization. For example, in CO2 (O=C=O), C forms 2 p-bonds and thus must have sp hybridization because this hybridization has two left-over p orbitals for the 2 p-bonds.
You can think about hybridzation and paint. Suppose you have four gallons of paint, one white (s) and three black (p) and are into mixing paint. You can mix two, three or four gallons together; it isn't necessary to use all four gallons. If the white and one black gallon are mixed, the result is two gallons of grey (two sp orbitals) and there are two gallons of black paint (two p orbitals) that were not used in the mixing. Note that the two gallons of grey have identical properties like color, smell, viscosity, etc. In the case of hydrid orbitals, an important difference between the two sp's is their orientation. They "point" in different directions and it is this property which gives rise to molecular shape.
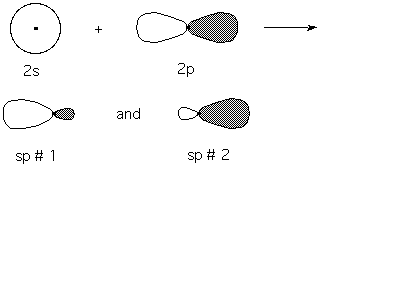
In the cartoon shown below the mixing of a 2s and one 2p AO gives 2 sp hydrid orbitals oriented in opposite directions. Note that most of the sp density is oriented in one direction (or the other). This allows the hybrid orbital to form bonds with more orbital overlap. More overlap translates into a better bond (stronger, lower E, more stable). With s and p AO's, half of the orbital density is pointing in the wrong direction for good s-bond formation.
