I. Two approaches to studying ecosystems.
A. How organisms transfer matter (e.g., competition, predation)
B. How matter flows through (treating organisms as boxes)
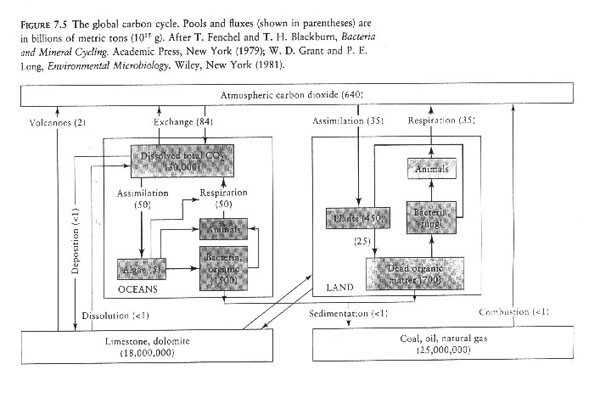
II. The Carbon cycle.

A. Where are the big pools?
1. Atmosphere
2. Rock and fossil fuesl
3. Dissolved in ocean
B. How does carbon flow among pools?
1. Biosphere --> plants
2. Exchange between water and atmosphere.
C. Biosphere has an enormous effect on carbon cycle.
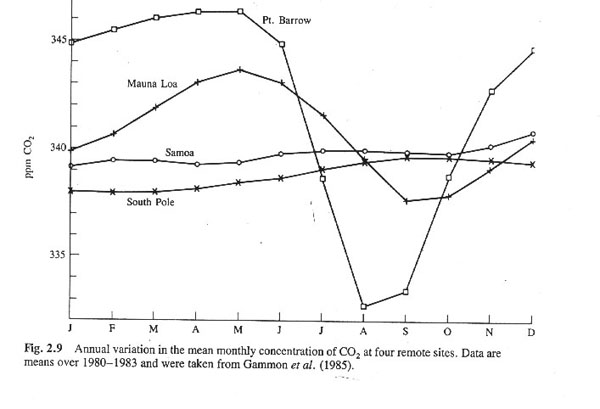
1. In spring in the arctic, one can measure the drawing down of carbon dioxide.
2. At the south pole or in Samoa, the flux is not very large.
3. At an observatory on Mauna Loa, which scientists consider to represent global changes, cycles are measurable.
III. Human effects on the carbon cycle.
A. Coal--> combustion--> CO2
1. < 1 billion metric tons in the 1930s
2. Today, burning of fossil fuels has increased
3. Also, deforestation also contributes to production of CO2 as wood is burned. One hectare (100m2) of trees has 50 tons of carbon.
IV. Consequences of changes in the carbon cycle: global climate change.
A. Changes are happening slowly and scientists need to predict outcomes of those changes. The degree of uncertainty varies a lot, from facts (statements so well supported, it would be perverse to argue) to hypotheses (proposed explanations) to predictions (logical outcomes if hypotheses and their assumptions are true).
B. Facts
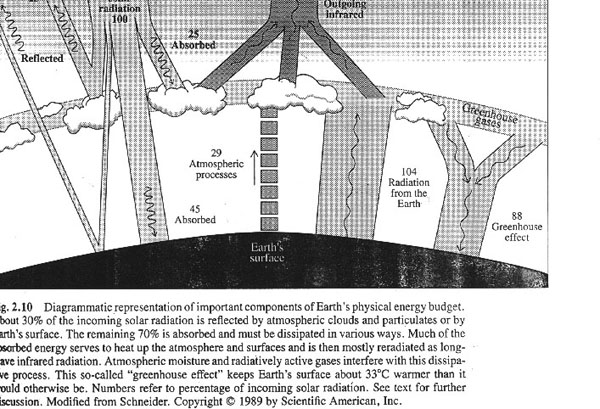
1. The Greenhouse effect is a fact from atmospheric science.
a. There is a natural greenhouse effect which keeps the Earth warm enough (average temperature about 60 o F) to be habitable.
b. Greenhouse gases like carbon dioxide, methane, and nitrous oxide and water vapor trap heat and warm the earth's surface
c. The basic principles of the greenhouse effect are well understood.
i. For a given concentration of greenhouse gases, the resulting amount of radiative forcing (or heat trapping of energy) can be predicted with precision.
ii. Exactly how the Earth's climate will respond to enhanced greenhouse gases will also depend on complex interactions between the atmosphere, oceans, land, ice, and biosphere.
2. Greenhouse gas emissions have increased.
a. In 1994, U.S. greenhouse gas emissions were about 1.65 billion metric tons of carbon (measured on a carbon equivalent basis) and represents about 20% of the global total.
b. Emissions of CO 2 accounted for the largest share of U.S.greenhouse gas emissions -- about 85% of the total.
c. Fossil fuel combustion is main source of CO 2 emissions.
d. Utilities, transportation, and industrial sectors are major sources of fossil fuel CO 2 emissions.
e. Future growth in CO 2 emissions is expected to be higher for developing countries.
3. Emissions of greenhouse gases are having an effect on carbon dioxide pool.
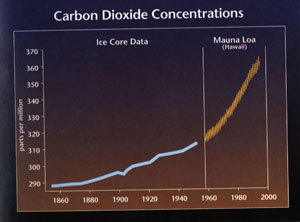
a. A clear trend of increasing CO2 from 1958 (315 ppm) to 1999 (370 ppm) is evident.
b. Ice age cores from prior to the industrial revolution also indicate rising levels of carbon dioxide.
4. The surface of the earth is getting warmer. (Where and what parts may still be controversial.)
5. Change in global average temperature is the norm.
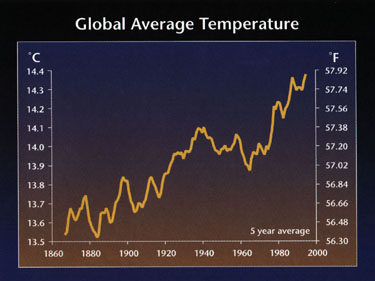
6. CO2 and temperature are correlated.
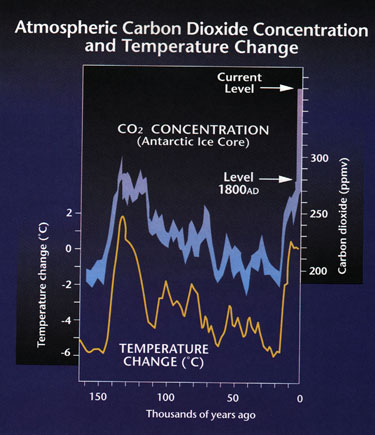
7. Current temperature and CO2 are at very high points
C. The hypotheses about global climate change: Human related increases of CO2 emission is causing rise in global temperature. Intergovernmental Panel on Climate
Change (IPCC) provides comprehensive, objective and balanced
assessments of climate change science and impacts and represents views of 2,500 of the world's leading climate scientists and technical experts. IPCC reported that evidence supported the idea that human caused emissions were having global effects on climate change and that hypothesis should be carefully considered.
D. The predictions.
1. CO2 levels will double.
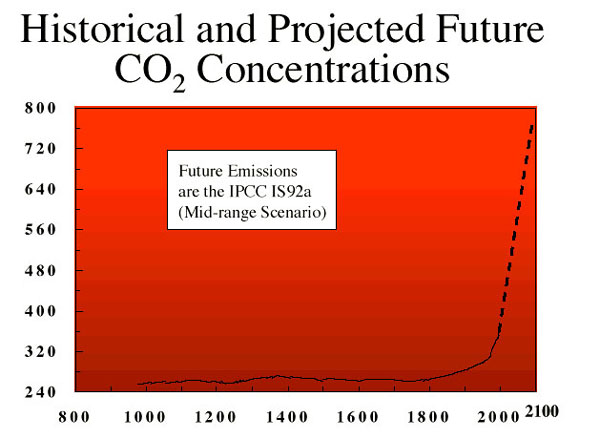
2. Models of climate change predict an 2.5 C increase in temperature.
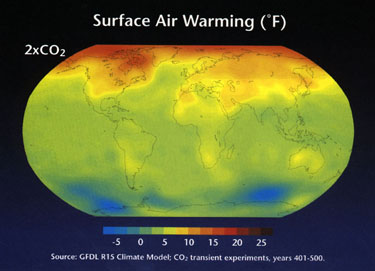
3. Changes will occur in precipitation.
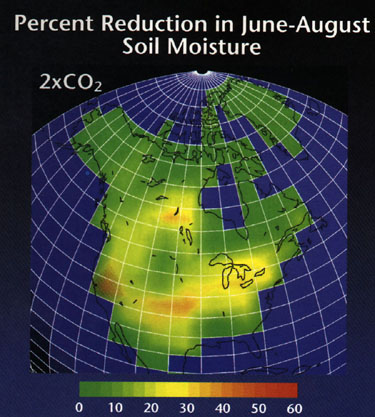
a. Increased evaporation.
b. Decreased water available in soils.
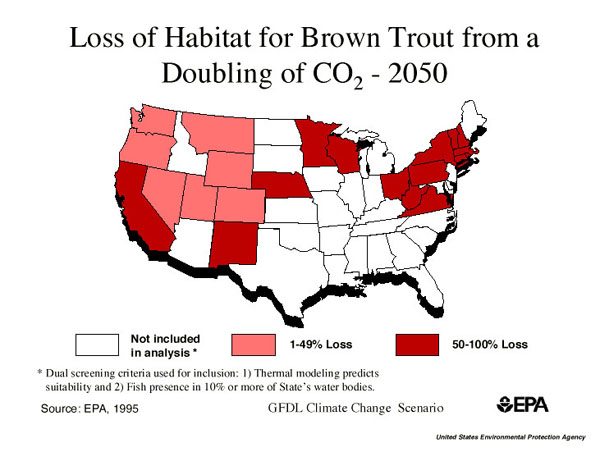
4. Effects on biology will include changes in biomes; recall importance of climate and moisture in defining different biomes.Such changes will have global effects on food production, harvesting of resources, as well as the quality of life.

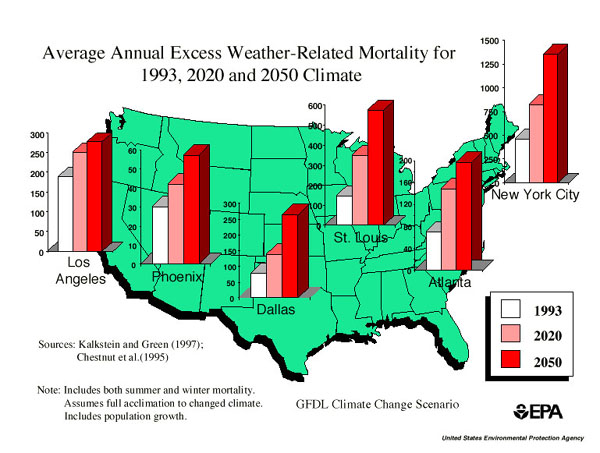
5. Human related effects will also accompany global climate change, such as increased heat related mortality.

V. In addition to Carbon, Nitrogen and Phosphorous are two other important elements. Nitrogen is in all amino acids, the building blocks of proteins. Phosphorous in used in nucleic acids, cell membranes, bones, and teeth.
VI. Nutrients, such as nitrogen and phosphorous are often limiting resources for primary producers.
VII. Understanding how nutrients cycle is important because it determines productivity in natural ecosystems, agriculture, aquatic systems, determines water quality. Also important in understanding acid rain, water pollution.
VIII. The nitrogen cycle.
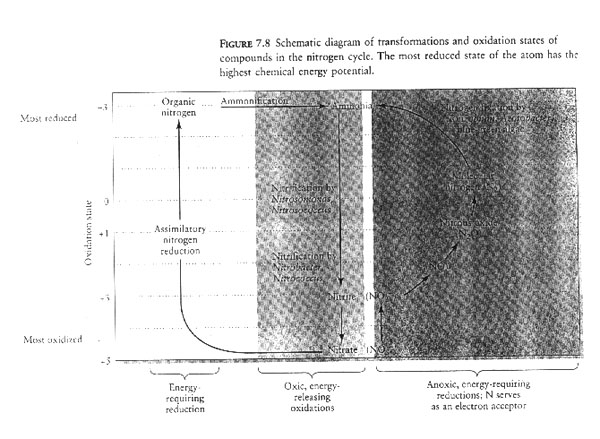
A. Atmospheric pool (atmosphere is 80% N). We cannot use N2 to make amino acids.
B. Nitrogen enters biological pathway through assimilation or nitrogen fixation (N2--> NH3) by very few microorganism, free-living bacteria Azotobacter, or symbiotic species Rhizobium which occurs in association with roots of some legumes and the blue-green cyanobacteria.These organisms use energy from metabolites such as sugars to reduce N to ammonia.
C. Animal waste and decomposition of plants also enter as ammonia.
D. Nitrification oxides nitrogen 1) from ammonia to nitrite (NH3 NO2-) by specialized bacteria (Nitrosomonas in soil, Nitrosococcus in marine systems) and 2) from nitrite to nitrate (NO3-)by Nitrobacter in the soil and Nitrococcus in marine.
E. Nitrates are what plants use; fertilizers are nitrates.
F. In waterlogged soils nitrate and nitrites are involved in denitrification by bacteria such as Psuedomonas denitrificans (NO3- --> NO2- --> NO) and eventually leads to production of molecular nitrogen (N2).
G. Nutrient losses in terrestrial communities channelled through streams. Comparing nutrients levels of incoming precipitation with nutrients in stream water is one approach to understanding uptake and cycling. (Ex. Hubbard Brook Experimental Forest).
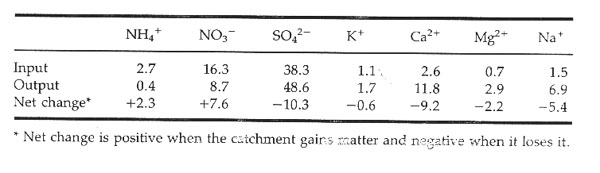
Stream output at Hubbard Brook represents only 0.1 % of the total organic nitrogen standing crop held in living and dead organic matter. Indicates that biological nitrogen cycle is very tight.
V. The phosphorous cycle.
A. Relatively simple since there is no atmospheric component.
B. Mineral pool isearth's crust and input into ecosystems occurs from weathering of rocks.
C. Plants have the metabolic means to absorb dissolved ionized forms of phosphorous (phosphate) and associations with microrhyzal fungi facilitate phosphate uptake.
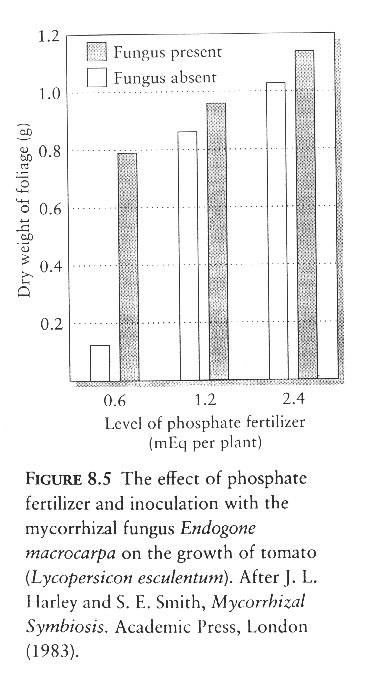
D. Plants efficiently and rapidly absorb phosphates and reduce soil concentrations to extremely low levels.
E. But phosphates bind tightly to Al, Fe (acid) Ca (alkaline) and clay compounds.
F. Animals obtain phosphates from what they eat.
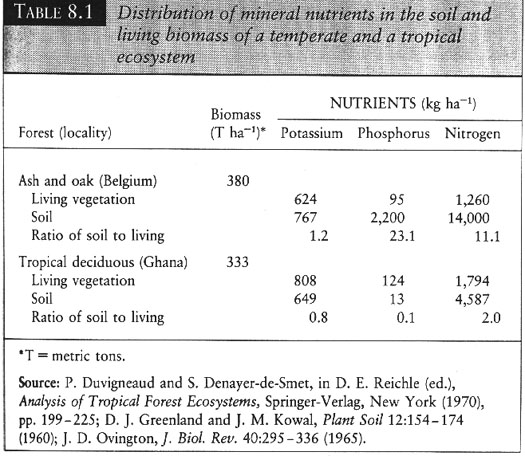
VI. Distribution of mineral nutrients differs among ecosystems.
A. In terrestrial habitats N limited, N water soluble and dissolves in water. N fixation slow due to slow gas exchange. P held in soil, sinks with clay and held in sediments.
B. In freshwater, P limited, N is more available due to runoff from soil, N fixation higher than terrestrial systems due to rapid gas exchange. Low runoff of P, since P sinks with clay and is held in sediments.
C. Even differences occur in terrestrial habitats.
VII. Human influences on nutrient cycling.
A. Eutrophication. Huge amounts of phosphates (10,000,000,000 kg) have been added to US waters. Phosphate detergents represented 30-40% entering aquatic systems. Also large inputs of nitrates have occurred from fertilizers. Why a problem? Cause algal blooms--leads to turbid water, decreases presence of macrophyta and associated organisms; decomposition of algae leads to anoxia at lower depths. In Chesapeake, eutrophication decreases fish and shellfish habitat.
B. Deforestation
1. Large scale experiments at Hubbard Brook, all the trees felled in a single catchment.
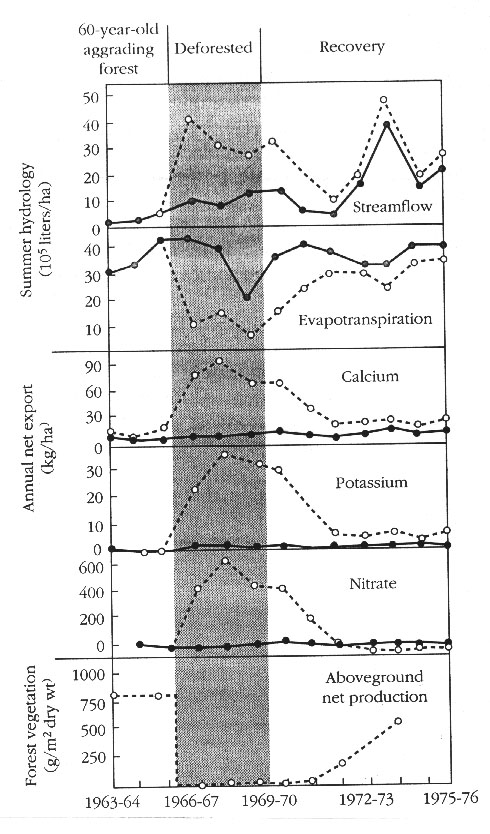
2. Overall rate of export of dissolved inorganic substances from disturbed catchment was 13x higher and can be explained by two factors.
a. Reduction in transpiration caused more precipitation to pass through soil and into streams.
b. Stream had more flow, more leaching and weathering. But also within system nutrient cycling broken, no plants to uptake nutrient and consequently are leached. Notice the main effect of deforestation on nitrate compared to other elements. This further emphasizes the tight cycling of nitrogen.
3. Nitrogen leached from land ends up in aquatic systems.
C. Acid deposition.
1. Nutrient availability depends on soil pH.
2. Mechanism of acid deposition. Additives to atmosphere SO2 and NOx
3. Sampling of rain pH relative to other substances.
| pH | |
| Pre-industrial Revolution rain | > 5 |
| "Pure" rain | 5.6 |
| "Natural" acid rain | 4.9 |
| Rain, Toronto, 1979 | 3.5 |
| Vinegar | 3.0 |
| Fog, Los Angeles, 1981 | 2.2 |
| Lemon juice | 2.2 |
| Rain, Wheeling, West Virginia | 1.4 |
| Battery acid | 1.0 |
4. Effects of acid rain on organisms.
a. In aquatic systems, affect osmoregulation, enzyme activity, gas exchange across gills, increase toxic metals, lead to community level effects (Ex. salmon in Nova Scotia rivers, Norwegian lakes in a 13,000 km2 area are fishless; In Sweden all bodies of water acidified, 15,000 too acidic for sensitive organisms, 1800 lifeless; In Adirondacks in 1930s 4% of lakes had pH <5, by 1970, 51% had. Fish wiped out--influences predators on fish such as loons.
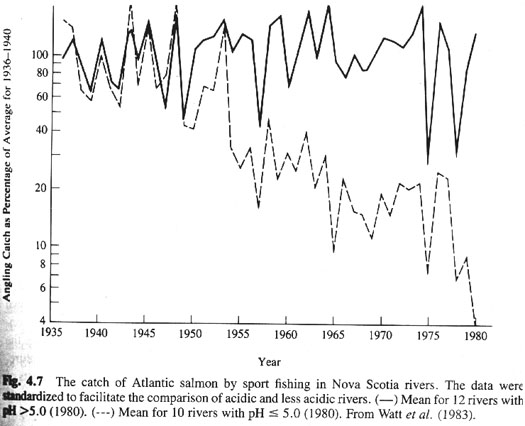
b. In terrestrial habitats, widespread damage and destruction of forests in Canada and Germany. Acid deposition breaks down lipids in foliage, damages membranes possibly leading to increased susceptibility to disease. Nutrients are leached out of the soil, damage to microrrhizae, and increase in toxic elements.
c. Good news :-) Regulation of emissions occurred by the Clean Air Act in 1970 and its stricter amendments in 1990. Sulfur emission reduced to 50% of 1980 levels by next year. Acid rain has abated
d.Bad news :-( Very slow recovery and emission regulations may not be strict enough. Vegetation in Hubbard Brook has nearly stopped growing since 1987; stream pH in Northeast remains below normal. Base cations such as calcium and magnesium leached away and no longer available for buffering. Using calculations for estimating input and output of calcium, Likens et al. estimated that the pool of calcium had shrunk by more than 50% in the last 45 years. Although loss has slowed in recent years, rock weathering won't replenish the pool of available calcium until well into the 21st century